Need help?
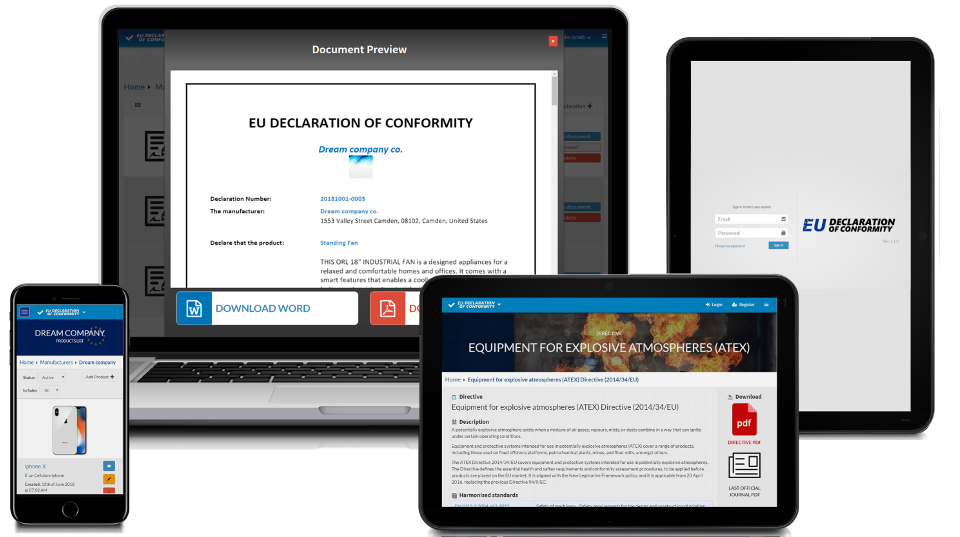
Your declarations of conformity online
This Free of Charge application was designed to help manufacturers, consultants, notified bodies to keep under control the EU declaration of conformity
This software allows you to compose in few minutes declaration of conformity compliant with applicable directives taking under control list of products, declarations of conformity, amended declarations
EN ISO 14971:2012 Standard


 Keywords:
Keywords:
 Directive
Directive
 Last Official Journal reference
Last Official Journal reference
 Description:
Description:
 Purpose
Purpose
 Publication
Publication
 Reference
Reference
Anyway is still possible use the "EN ISO 14971:2009" until August 30th, 2012

Active implantable medical devices Directive (90/385/EEC)
Biological evaluation of medical devices - Part 12: Sample preparation and reference materials (ISO 10993-12:2012)

Active implantable medical devices Directive (90/385/EEC)
Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity (ISO 10993-5:2009)

Active implantable medical devices Directive (90/385/EEC)
Sterilization of medical devices - Microbiological methods - Part 1: Determination of a population of microorganisms on products (...

Active implantable medical devices Directive (90/385/EEC)
Packaging for terminally sterilized medical devices - Part 1: Requirements for materials, sterile barrier systems and packaging sy...

Active implantable medical devices Directive (90/385/EEC)
Sterilization of health care products - Radiation - Part 1: Requirements for development, validation and routine control of a ster...

Active implantable medical devices Directive (90/385/EEC)
Sterilization of health care products - Ethylene oxide - Part 1: Requirements for development, validation and routine control of a...
SITEMAP
OTHER APPLICATIONS
If you are interested in DO160 tests, you can give a try to our other free of charge software:
This Free of Charge application was designed to help manufacturers, consultants, notified bodies to keep under control the EU declaration of conformity
This software allows you to compose in few minutes declaration of conformity compliant with applicable directives taking under control list of products, declarations of conformity, amended declarations

We and selected third parties use cookies or similar technologies for technical purposes and, with your consent, for “basic interactions & functionalities”, “experience enhancement”, “measurement” and “targeting & advertising”. Denying consent may make related features unavailable.
You can freely give or deny your consent

This Application collects some Personal Data from its Users.
Among the types of Personal Data that this Application collects, by itself or through third parties, there are: Cookies; Usage Data.
Complete details on each type of Personal Data collected are provided in the dedicated sections of this privacy policy or by specific explanation texts displayed prior to the Data collection.
Personal Data may be freely provided by the User, or, in case of Usage Data, collected automatically when using this Application.
Unless specified otherwise, all Data requested by this Application is mandatory and failure to provide this Data may make it impossible for this Application to provide its services. In cases where this Application specifically states that some Data is not mandatory, Users are free not to communicate this Data without consequences to the availability or the functioning of the Service.
Users who are uncertain about which Personal Data is mandatory are welcome to contact the Owner.
Any use of Cookies – or of other tracking tools – by this Application or by the owners of third-party services used by this Application serves the purpose of providing the Service required by the User, in addition to any other purposes described in the present document and in the Cookie Policy, if available.
Users are responsible for any third-party Personal Data obtained, published or shared through this Application and confirm that they have the third party's consent to provide the Data to the Owner.
The Owner takes appropriate security measures to prevent unauthorized access, disclosure, modification, or unauthorized destruction of the Data.
The Data processing is carried out using computers and/or IT enabled tools, following organizational procedures and modes strictly related to the purposes indicated. In addition to the Owner, in some cases, the Data may be accessible to certain types of persons in charge, involved with the operation of this Application (administration, sales, marketing, legal, system administration) or external parties (such as third-party technical service providers, mail carriers, hosting providers, IT companies, communications agencies) appointed, if necessary, as Data Processors by the Owner. The updated list of these parties may be requested from the Owner at any time.
Legal basis of processingThe Owner may process Personal Data relating to Users if one of the following applies:
In any case, the Owner will gladly help to clarify the specific legal basis that applies to the processing, and in particular whether the provision of Personal Data is a statutory or contractual requirement, or a requirement necessary to enter into a contract.
PlaceThe Data is processed at the Owner's operating offices and in any other places where the parties involved in the processing are located.
Depending on the User's location, data transfers may involve transferring the User's Data to a country other than their own. To find out more about the place of processing of such transferred Data, Users can check the section containing details about the processing of Personal Data.
Users are also entitled to learn about the legal basis of Data transfers to a country outside the European Union or to any international organization governed by public international law or set up by two or more countries, such as the UN, and about the security measures taken by the Owner to safeguard their Data.
If any such transfer takes place, Users can find out more by checking the relevant sections of this document or inquire with the Owner using the information provided in the contact section.
Retention timePersonal Data shall be processed and stored for as long as required by the purpose they have been collected for.
Therefore:
The Owner may be allowed to retain Personal Data for a longer period whenever the User has given consent to such processing, as long as such consent is not withdrawn. Furthermore, the Owner may be obliged to retain Personal Data for a longer period whenever required to do so for the performance of a legal obligation or upon order of an authority.
Once the retention period expires, Personal Data shall be deleted. Therefore, the right to access, the right to erasure, the right to rectification and the right to data portability cannot be enforced after expiration of the retention period.
The Data concerning the User is collected to allow the Owner to provide its Services, as well as for the following purposes: Analytics and SPAM protection.
Users can find further detailed information about such purposes of processing and about the specific Personal Data used for each purpose in the respective sections of this document.
Personal Data is collected for the following purposes and using the following services:
The services contained in this section enable the Owner to monitor and analyze web traffic and can be used to keep track of User behavior.
Google Analytics (Google Ireland Limited)
Google Analytics is a web analysis service provided by Google Ireland Limited (“Google”). Google utilizes the Data collected to track and examine the use of this Application, to prepare reports on its activities and share them with other Google services.
Google may use the Data collected to contextualize and personalize the ads of its own advertising network.
Personal Data collected: Cookies; Usage Data.
Place of processing: Ireland – Privacy Policy. Privacy Shield participant.
This type of service analyzes the traffic of this Application, potentially containing Users' Personal Data, with the purpose of filtering it from parts of traffic, messages and content that are recognized as SPAM.
Google reCAPTCHA (Google Ireland Limited)
Google reCAPTCHA is a SPAM protection service provided by Google Ireland Limited.
The use of reCAPTCHA is subject to the Google privacy policy and terms of use.
Personal Data collected: Cookies; Usage Data.
Place of processing: Ireland – Privacy Policy. Privacy Shield participant.
Users may exercise certain rights regarding their Data processed by the Owner.
In particular, Users have the right to do the following:
Where Personal Data is processed for a public interest, in the exercise of an official authority vested in the Owner or for the purposes of the legitimate interests pursued by the Owner, Users may object to such processing by providing a ground related to their particular situation to justify the objection.
Users must know that, however, should their Personal Data be processed for direct marketing purposes, they can object to that processing at any time without providing any justification. To learn, whether the Owner is processing Personal Data for direct marketing purposes, Users may refer to the relevant sections of this document.
How to exercise these rightsAny requests to exercise User rights can be directed to the Owner through the contact details provided in this document. These requests can be exercised free of charge and will be addressed by the Owner as early as possible and always within one month.
The User's Personal Data may be used for legal purposes by the Owner in Court or in the stages leading to possible legal action arising from improper use of this Application or the related Services.
The User declares to be aware that the Owner may be required to reveal personal data upon request of public authorities.
In addition to the information contained in this privacy policy, this Application may provide the User with additional and contextual information concerning particular Services or the collection and processing of Personal Data upon request.
System logs and maintenanceFor operation and maintenance purposes, this Application and any third-party services may collect files that record interaction with this Application (System logs) use other Personal Data (such as the IP Address) for this purpose.
Information not contained in this policyMore details concerning the collection or processing of Personal Data may be requested from the Owner at any time. Please see the contact information at the beginning of this document.
How “Do Not Track” requests are handledThis Application does not support “Do Not Track” requests.
To determine whether any of the third-party services it uses honor the “Do Not Track” requests, please read their privacy policies.
The Owner reserves the right to make changes to this privacy policy at any time by giving notice to its Users on this page and possibly within this Application and/or - as far as technically and legally feasible - sending a notice to Users via any contact information available to the Owner. It is strongly recommended to check this page often, referring to the date of the last modification listed at the bottom.
Should the changes affect processing activities performed on the basis of the User’s consent, the Owner shall collect new consent from the User, where required.
Any information regarding a natural person, a legal person, an institution or an association, which is, or can be, identified, even indirectly, by reference to any other information, including a personal identification number.
Usage DataInformation collected automatically through this Application (or third-party services employed in this Application), which can include: the IP addresses or domain names of the computers utilized by the Users who use this Application, the URI addresses (Uniform Resource Identifier), the time of the request, the method utilized to submit the request to the server, the size of the file received in response, the numerical code indicating the status of the server's answer (successful outcome, error, etc.), the country of origin, the features of the browser and the operating system utilized by the User, the various time details per visit (e.g., the time spent on each page within the Application) and the details about the path followed within the Application with special reference to the sequence of pages visited, and other parameters about the device operating system and/or the User's IT environment.
UserThe individual using this Application who, unless otherwise specified, coincides with the Data Subject.
Data SubjectThe natural person to whom the Personal Data refers.
Data Processor (or Data Supervisor)The natural or legal person, public authority, agency or other body which processes Personal Data on behalf of the Controller, as described in this privacy policy.
Data Controller (or Owner)The natural or legal person, public authority, agency or other body which, alone or jointly with others, determines the purposes and means of the processing of Personal Data, including the security measures concerning the operation and use of this Application. The Data Controller, unless otherwise specified, is the Owner of this Application.
This ApplicationThe means by which the Personal Data of the User is collected and processed.
ServiceThe service provided by this Application as described in the relative terms (if available) and on this site/application.
European Union (or EU)Unless otherwise specified, all references made within this document to the European Union include all current member states to the European Union and the European Economic Area.
This privacy policy relates solely to this Application.
CookiesSmall sets of data stored in the User's device.